Dutasteride : New drug used to treatment male baldness

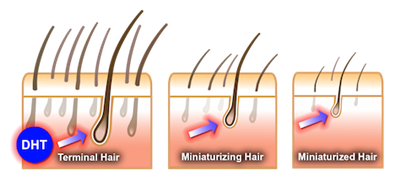
Dutasteride (Avodart), , is a dual 5-α reductase inhibitor that inhibits conversion of testosterone to dihydrotestosterone (DHT). It is used to treat benign prostatic hyperplasia. It increases the risk of erectile dysfunctionand decreased sexual desire.
Mechanism of action
Dutasteride belongs to a class of drugs called 5-alpha-reductase inhibitors, which block the action of the 5-alpha-reductase enzymes that convert testosterone into dihydrotestosterone (DHT).
Medical uses
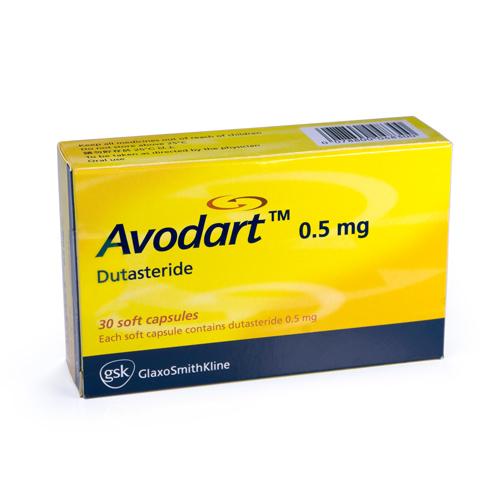
Avodart (dutasteride) 500 µg soft capsules.Dutasteride is useful for treating benign prostatic hyperplasia (BPH); colloquially known as an “enlarged prostate”.
In those who are being regularly screened, 5-alpha-reductase inhibitors such as finasteride and dutasteride reduce the overall risk of being diagnosed with prostate cancer; however, there is insufficient data to determine if they have an effect on the risk of death and may increase the chance of more serious cases
Dutasteride versus finasteride
Finasteride is also approved for the treatment of benign prostatic hyperplasia, or BPH. The medications belong to the same class of drugs.
Dutasteride inhibits two of the three isoforms of 5-alpha reductase, I and II, whereas finasteride only inhibits type II, and has a much shorter half-life. Bexlosteride is an isoform I selective inhibitor,albeit with limited commercial availability
Adverse effects
1. Sexual effects This class of medications increases rates of erectile dysfunction (with between 5% and 9% developing problems after starting their use).This is linked to lower quality of life and can cause stress in relationships.There is also an association with lowered sexual desire.It has been reported that these adverse sexual side effects may persist even after discontinuation of the drug.
2. Prostate cancer The FDA has added a warning to dutasteride about an increased risk of high-grade prostate cancer.While the potential for positive, negative or neutral changes to the potential risk of developing prostate cancer with dutasteride has not been established, evidence has suggested it may temporarily reduce the growth and prevalence of benign prostate tumors, but could also mask the early detection of prostate cancer. The primary area for concern is for patients who may develop prostate cancer whilst taking dutasteride for benign prostatic hyperplasia, which in turn could delay diagnosis and early treatment of the prostate cancer, thereby potentially increasing the risk of these patients developing high-grade prostate cancer.
Contraindications
Children and women who are or may become pregnant, and people with known significant hypersensitivity (e.g., serious skin reactions, angioedema) to dutasteride or finasteride should not take dutasteride. Exposure to dutasteride and other 5-alpha reductase inhibitors during pregnancy can cause birth defects. Since these medications are readily absorbed through the skin, women who are or may become pregnant should not handle them and if they come into contact with leaking capsules, the contact area should be washed immediately in soapy water. People taking dutasteride should not donate blood and, due to its long half-life, should also not donate blood for at least 6 months after the cessation of treatment.
Related
Laser Hair Treatment : LOW-LEVEL LASER THERAPY (LLLT) for Hair Loss
ABOUT LOW-LEVEL LASER THERAPY (LLLT) Low-Level Laser Light is one of the most effective therapies to treat hair loss. Currently, over-the-counter laser therapy devices for hair growth are available through convenient hands-free systems, or less convenient hand-held devices. Either product may be used at home, and does not require the supervision of a physician. LLLT ...